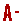
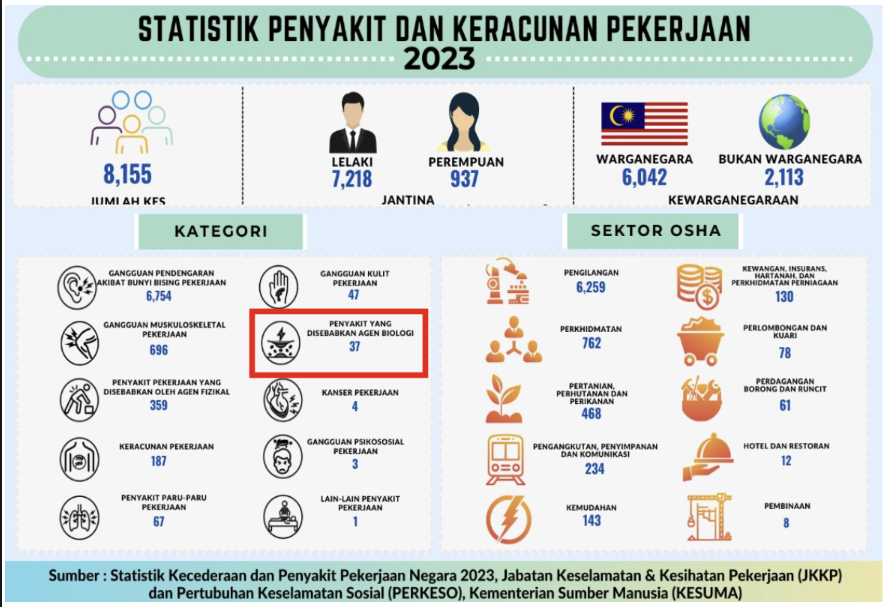
March, 7 – The Institute of Plantation Studies (IKP) organized a Biosecurity and Biosafety Awareness Briefing on March 6, 2025, at the IKP Meeting Room. The briefing was presented by Mr. Mohd. Azman bin Ahmad from the Institutional Biosafety and Biosecurity Committee (IBBC), Occupational Safety and Health Management Office, UPM, and attended by 16 IKP staff members from the management, research, and laboratory management sections. Mr. Azman provided further explanations on the importance of monitoring activities related to genetically modified microorganisms, including biological agents such as living modified organisms (LMO), genetically modified organisms (GMO), rDNA, and biological toxins, as well as the procedures involved. This is to ensure that all activities related to LMO, GMO, rDNA, and biological toxins in all facilities, including teaching, research, clinical, veterinary services, and environmental laboratories, comply with the Biosafety Act 2007. Researchers directly involved with studies involving biological materials are required to ensure all activities are conducted safely, including establishing related SOPs (administrative control), using Biosafety Cabinets (engineering control), implementing Good Laboratory Work Practices (GLWP), and utilizing Personal Protective Equipment (PPE) to reduce the risk of exposure to biological materials that may cause infections or accidents. Researchers must also ensure that all individuals involved in these projects are competent in handling animals. Mr. Azman also emphasized that non-compliance with biosafety and biosecurity practices exposes researchers to legal actions despite its impacts to the environment, as well as poses a threat to public health.

Date of Input: 19/03/2025 |
Updated: 19/03/2025 | ainzubaidah
MEDIA SHARING